Fig. 3
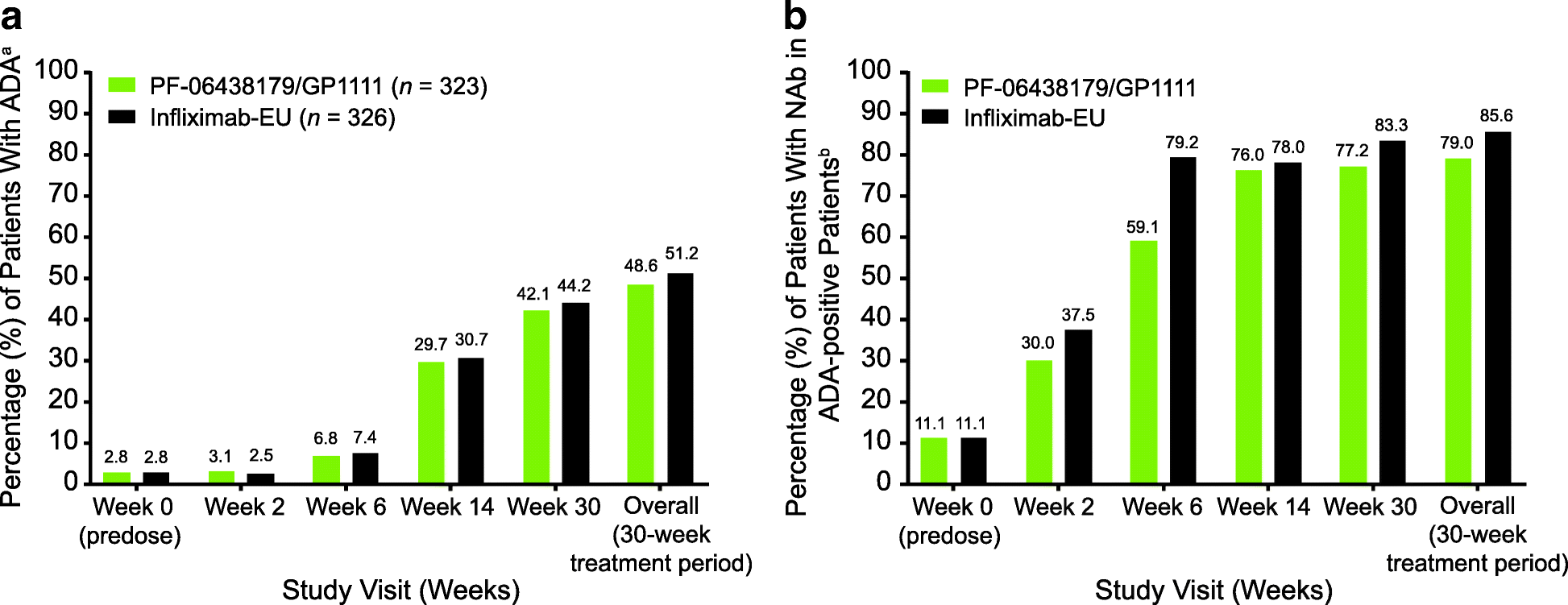
ADA and NAb incidence by study visit (safety population). a ADA incidence. b NAb incidence. aADA-positive and ADA-negative test results were defined as ADA titer ≥ 1.30 and < 1.30, respectively. Overall, a patient who tested positive was defined as having at least one postdose positive sample during the 30-week treatment period, regardless of predose ADA status. bNAb-positive and NAb-negative results were defined as NAb titer ≥ 0.70 and < 0.70, respectively. Incidences of NAb-positive patients are expressed as percentages of ADA-positive patients. ADA Antidrug antibody; Infliximab-EU Infliximab sourced from the European Union, NAb, Neutralizing antibody