- Research
- Open access
- Published:
Effectiveness of janus kinase inhibitors in relapsing giant cell arteritis in real-world clinical practice and review of the literature
Arthritis Research & Therapy volume 26, Article number: 116 (2024)
Abstract
Background
A substantial proportion of patients with giant cell arteritis (GCA) relapse despite standard therapy with glucocorticoids, methotrexate and tocilizumab. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway is involved in the pathogenesis of GCA and JAK inhibitors (JAKi) could be a therapeutic alternative. We evaluated the effectiveness of JAKi in relapsing GCA patients in a real-world setting and reviewed available literature.
Methods
Retrospective analysis of GCA patients treated with JAKi for relapsing disease at thirteen centers in Spain and one center in United States (01/2017-12/2022). Outcomes assessed included clinical remission, complete remission and safety. Clinical remission was defined as the absence of GCA signs and symptoms regardless of the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values. Complete remission was defined as the absence of GCA signs and symptoms along with normal ESR and CRP values. A systematic literature search for other JAKi-treated GCA cases was conducted.
Results
Thirty-five patients (86% females, mean age 72.3) with relapsing GCA received JAKi therapy (baricitinib, n = 15; tofacitinib, n = 10; upadacitinib, n = 10). Before JAKi therapy, 22 (63%) patients had received conventional synthetic immunosuppressants (e.g., methotrexate), and 30 (86%) biologics (e.g., tocilizumab). After a median (IQR) follow-up of 11 (6-15.5) months, 20 (57%) patients achieved and maintained clinical remission, 16 (46%) patients achieved and maintained complete remission, and 15 (43%) patients discontinued the initial JAKi due to relapse (n = 11 [31%]) or serious adverse events (n = 4 [11%]). A literature search identified another 36 JAKi-treated GCA cases with clinical improvement reported for the majority of them.
Conclusions
This real-world analysis and literature review suggest that JAKi could be effective in GCA, including in patients failing established glucocorticoid-sparing therapies such as tocilizumab and methotrexate. A phase III randomized controlled trial of upadacitinib is currently ongoing (ClinicalTrials.gov ID NCT03725202).
Introduction
Giant cell arteritis (GCA) is an inflammatory disorder of large and medium-sized arteries affecting people over 50 years [1, 2]. It is the most common type of vasculitis in adults in Europe and North America. The disease is characterized by the granulomatous involvement of the aorta and its main branches with complications including blindness and thoracic aortic aneurysm [1, 2].
Glucocorticoids have been the cornerstone of GCA treatment for decades at the expense of significant treatment-related toxicity and high relapse rates upon dose reduction or drug discontinuation. Other therapies, such as methotrexate, leflunomide, azathioprine, hydroxychloroquine, cyclophosphamide or tumor necrosis factor (TNF)-\(a\) inhibitors have proven to be ineffective or shown mixed results [3, 4]. To date, tocilizumab, a monoclonal antibody against the IL-6 receptor (IL-6R), is the only medication with demonstrated efficacy in terms of remission maintenance and glucocorticoid-sparing [5, 6]. However, up to 40% of patients receiving tocilizumab fail treatment due to disease relapse or tocilizumab-related side effects [6, 7]. In addition, more than half of the patients responding to tocilizumab relapse upon drug discontinuation [6, 8,9,10]. Therefore, other treatment options are greatly needed for patients with GCA.
Advances in the understanding of the pathophysiology of GCA have paved the way for several therapies that are currently under investigation [11,12,13]. In the last years, the critical role of the janus kinase/signal transducers and activators of transcription (JAK/STAT) pathways in immune-mediated diseases has been therapeutically exploited with the development of JAK inhibitors (JAKi), small molecules that block the action of type I/II cytokines [14]. In GCA, CD4+ T-cells and macrophages, which respond to certain key mediators through the JAK/STAT system (e.g., IL-6/STAT3, granulocyte-macrophage colony-stimulating factor [GM-CSF]/STAT5, interferon [IFN]-γ/STAT1), are present in the arterial inflammatory lesions [14]. Thus, the inhibition of JAK signalling could be an effective treatment as suggested in animal models [14]. Nevertheless, the published literature on the utility of JAKi in GCA is scarce consisting in retrospective case reports and small series of patients, and a single prospective pilot study of 15 patients [15,16,17,18,19,20]. Therefore, additional data on this topic would be valuable.
We retrospectively assessed the outcomes of a series of patients with relapsing GCA treated with JAKi in a real-world setting. We also systematically searched the literature for other patients treated with JAKi and compared the group of patients in our series receiving baricitinib with patients that received this medication in the setting of the pilot study mentioned above [15].
Methods
Study design and patient population
We conducted an observational, retrospective analysis of patients with GCA treated with JAKi in thirteen centers in Spain and one center in United States. All patients met the 1990 American College of Rheumatology classification criteria for GCA [21], and/or had positive temporal artery biopsy or evidence suggesting vasculitis by imaging. The types of vascular imaging studies considered for the purpose of GCA diagnosis were ultrasound of the temporal arteries (i.e., halo sign), and computed tomography angiography (CTA) (i.e., diffuse, concentric mural thickening), magnetic resonance imaging/angiography (MRI/MRA) (i.e., diffuse, concentric mural thickening with or without T2 hyperintensity and/or contrast uptake), and positron emission tomography (PET) (e.g., diffuse, concentric F18 fluorodeoxyglucose [FDG] uptake) of the large arteries (e.g., aorta and main aortic branches).
Patients received JAKi at the discretion of the treating rheumatologist for disease relapsed despite the use of glucocorticoids (e.g., prednisone or methylprednisolone) and other immunosuppressants including conventional synthetic (e.g., methotrexate) and biologic (e.g., tocilizumab) immunosuppressants. A washout period corresponding to a half-life of elimination of the biologic agent was carried out between the end of biologic therapy and the start of JAKi therapy. Because this was a retrospective study of real-world data generated by a group of independent rheumatologists, there was no pre-determined criteria to select the type or dose of JAKi or the glucocorticoid tapering regimen following JAKi therapy initiation. Factors influencing providers when making those decisions may have included patient’s preference, provider’s experience and judgement, insurance authorization, cost, and safety.
Study assessments and outcomes
Effectiveness and safety outcomes were evaluated during JAKi treatment by systematically reviewing all rheumatology notes, laboratory values and vascular imaging results available in each patient’s medical record. During follow-up, patients were seen by the rheumatology providers at variable intervals, but mostly every one to six months. Data were extracted from the medical records following a specifically designed protocol. To minimize entry mistakes, all data were double-checked.
The primary outcome was clinical remission defined as the absence of signs and symptoms attributable to GCA (e.g., headaches, jaw claudication, polymyalgia rheumatica symptoms [PMR], etc.) regardless of the value of the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Secondary outcomes included complete remission defined, as per the European Alliance of Associations for Rheumatology (EULAR) criteria, as the absence of signs and symptoms attributable to GCA and the normalization of the ESR and CRP values [22]. Relapse was defined as the reappearance of clinical manifestations of GCA that required treatment intensification [23]. Additional outcomes evaluated were the ability to discontinue glucocorticoids and the occurrence of adverse events.
The ESR was considered to be increased when it was higher than 20 or 25 mm/hour for men or women, respectively. A serum CRP value greater than 0.5 mg/dL was considered elevated. Anemia was defined as a hemoglobin level ≤ 11 g/dL, leukopenia as < 4000 leukocytes/µL, lymphopenia as < 1500 lymphocytes/µL, neutropenia as < 1500 neutrophils/µL, and thrombocytopenia as < 100,000 platelets/µL.
Systematic literature search
A systematic literature search of published randomized controlled trials, non-randomized trials, cohort studies, case series, and case reports was done in MEDLINE/PubMed (https://pubmed.ncbi.nlm.nih.gov/) from the inception of each database to May 31, 2023.
Statistical analysis
All data were analyzed using descriptive methods. Continuous data were summarized using means, medians, standard deviations (SD), ranges and interquartile ranges (IQR) where appropriate. Categorical data were summarized as numbers and corresponding percentages. Additionally, a comparison between 15 GCA patients from a pilot study by Koster et al. [15] and the 15 GCA patients of our series treated with baricitinib was performed. Continues variables were compared using Mann-Whitney U-test and categorical variables were compared using Fisher´s exact test. Statistical significance was considered as a p-value < 0.05. The analysis was conducted using STATISTICA software (StatSoft Inc. Tulsa, OK, USA).
Ethical considerations
The study was approved by the Cantabria Clinical Research Ethics Comittee (approval number 2021.414), and was conducted in accordance with the Declaration of Helsinki and the International Conference for Harmonization. All data extracted from the medical records were stored de-identified prior to the analysis. As per the Clinical Research Ethics Committee, this retrospective research did not require informed consent.
Results
Baseline general features at JAKi initiation
A total of 35 patients (30 women and 5 men) with GCA who received treatment with JAKi were included. GCA was confirmed by temporal artery biopsy in 15 (62%) patients and by vascular imaging in 24 (69%) patients [Table 1]. Vascular ultrasonography was performed in 15 patients, observing signs of vasculitis in 7 of them. The mean (SD) age at the initiation of JAKi therapy was 72.3 (8.0) years. Overall, 15 (43%) patients received baricitinib (2–4 mg daily), 10 (29%) tofacitinib (5 mg twice a day) and 10 (29%) upadacitinib (15 mg daily). The median (IQR) time from GCA diagnosis to JAKi therapy initiation was 30 (12–48) months. Without considering concomitant glucocorticoid use, JAKi was prescribed as monotherapy in 34 (97%) patients, and combined with methotrexate in one patient. Thus, only one patient who started treatment with baricitinib maintained concurrent treatment with methotrexate at a dose of 10 mg weekly and a prednisone dose of 5 mg/day. Thirty-one patients started treatment with JAKi in combination with glucocorticoids, and three patients initiated JAKi therapy without any other drugs for the treatment of GCA.
The main clinical manifestations of the patients at the time of JAKi initiation are summarized in Table 1. Those included headache (n = 15 [43%]), jaw claudication (n = 6 [17%]), visual symptoms (n = 5 [14%]), and PMR symptoms (n = 12 [34%]). The median (IQR) baseline serum CRP and ESR values were 0.9 (0.4–2.5) mg/dL and 28 (7–48) mm/hour. The median (IQR) baseline prednisone dose was 16.2 (8.7–30) mg/day.
Before JAKi therapy, 22 (63%) patients had received several conventional synthetic immunosuppressants such as methotrexate (n = 22 [63%]), hydroxychloroquine (n = 3 [9%]), and leflunomide (n = 1 [3%]) [Table 1]. In addition, 30 (86%) patients had been treated with biologics including tocilizumab (n = 26 [74%]), sarilumab (n = 3 [9%]), abatacept (n = 8 [23%]), adalimumab (n = 2 [6%]), and ustekinumab (n = 2 [6%]) [Table 1].
Clinical outcomes
Once on JAKi, patients were followed for a median (IQR) period of 11 (6-15.5) months with 35 patients followed for at least one month, 33 patients followed for at least three months, 28 patients followed for at least six months and 20 patients followed for at least twelve months. Most patients experienced improvement of clinical manifestations and laboratory parameters over time following JAKi therapy. Clinical remission was observed at one, three, six and twelve months in 18/35 (51%), 18/33 (54%), 17/28 (61%) and 14/20 (70%) patients, respectively [Fig. 1A]. Complete remission was observed at one, three, six and twelve months in 15/35 (43%), 16/33 (48%), 16/28 (57%) and 13/20 (65%) patients, respectively [Fig. 1B]. Effectiveness was similar across all JAKi [Fig. 1A and B].
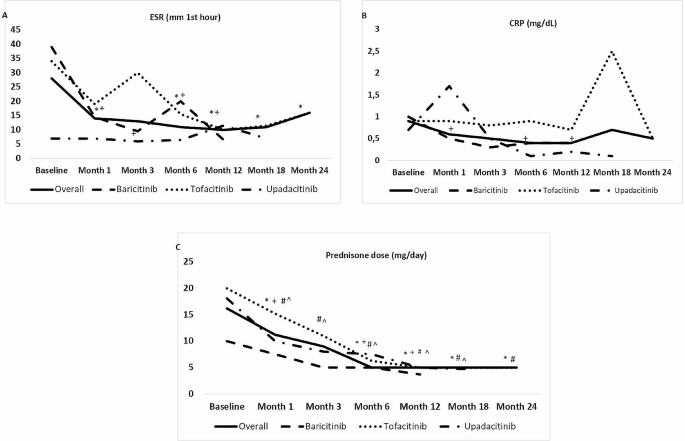
The median (IQR) ESR declined from 28 (7–48) mm/hour at baseline to 3 (7.2–29.2) mm/hour at last follow-up (p < 0.001). The median (IQR) CRP concentration decreased from 0.9 (0.4–2.5) at baseline to 0.4 (0.2–2.1) mg/dL at last follow up (p = 0.53) [Fig. 2A and B]. The median (IQR) daily dose of prednisone decreased from 16.2 (8.7–30) at baseline to 5 (0-12.5) mg at last follow up (p < 0.001) [Fig. 2C]. In addition, 7 (20%) patients were able to stop glucocorticoids completely.
Laboratory abnormalities and reduction of glucocorticoid dose after JAK inhibitor initiation
Legend: (data expressed as median values; p compared with baseline). (A) Erythrocyte sedimentation rate (ESR); (B) Serum C-reactive protein (CRP); and (C) Glucocorticoid dose. JAK: Janus kinase. *: p < 0.05 in overall series. +: p < 0.05 in baricitinib group. #: p < 0.05 in tofacitinib group. ^: p < 0.05 in upadacitinib group
Overall, eleven (31%) patients discontinued JAKi therapy due to relapse or persistence of active disease. Of these eleven patients, five were on tofacitinib, four on baricitinib, and two on upadacitinib.
Safety
Adverse events were reported in five (14%) patients during JAKi therapy. One patient on baricitinib developed a urinary tract infection without requiring permanent JAKi discontinuation. The adverse events led to drug discontinuation in the other four patients. These cases included a 74-year-old woman on baricitinib 2 mg/day that developed significant elevation of liver enzymes, a 67-year-old woman on tofacitinib 5 mg twice a day that developed palpitations and dyspnea, a 67-year-old woman on upadacitinib 15 mg daily complicated with disseminated herpes zoster, and a 72-year-old man diagnosed with glioblastoma multiforme six months after starting upadacitinib 15 mg daily. No thromboembolism, major adverse cardiovascular events, or significant cytopenias were observed during follow-up. No cases of GCA-related permanent vision loss were reported either.
Outcomes in patients previously treated with IL-6R antagonists
Overall, 28 (80%) patients (24 women and 4 men) had previously received treatment with IL-6R blockers including tocilizumab (n = 26) and sarilumab (n = 3) (one patient received both). Within this group of 28 patients, IL-6R blockade therapy had been stopped due to inefficacy in 20 (71%) of them.
The JAKi prescribed to this subgroup of patients were baricitinib (n = 10), tofacitinib (n = 9), and upadacitinib (n = 9). During a median (IQR) follow-up of 12 (8.7–16.2) months, 16 (57%) out of 28 patients who had previously received IL-6R blockers achieved clinical remission (baricitinib [n = 7; 44%], tofacitinib [n = 5; 31%], upadacitinib [n = 4; 25%]), and 13 (46%) of them complete remission (baricitinib [n = 6; 46%], tofacitinib [n = 3; 23%], upadacitinib [n = 4; 31%]), no observing differences between the three JAKi used. As expected, the ESR (median [IQR] 11 [5–39] mm/hour) and CRP (median [IQR] 0.8 mg/dL [0.3–1.9]) values at the moment of IL-6R blockade therapy discontinuation and initiation of JAKi were normal. Those values further decreased during JAKi therapy (median [IQR] ESR 10 [5.7–22] mm/hour and CRP 0.4 [0.1–1.2] at last follow up, p > 0.05 for both comparisons) At the last visit, the median (IQR) dose of prednisone decreased from 15.6 (10–30) mg/day to 5 (0.6–10) mg/day (p < 0.001). Of 25 patients who were receiving glucocorticoids at JAKi initiation, six (24%) were able to discontinue them. The median (IQR) daily prednisone dose at last follow up was 5 [0.6–10] mg. JAKi was withdrawn because of GCA relapse in nine (32%) patients. Of these, five patients were receiving tofacitinib, two baricitinib, and 2 upadacitinib.
Comparison between patients treated with baricitinib in a prospective pilot study and this case series
The main features of the two series of patients are summarized in Supplementary Table 1. The study by Koster et al. was a prospective, proof-of-concept trial of baricitinib (4 mg/day) that enrolled 15 GCA patients with relapsing disease, and employed a relatively rapid (15–22 weeks), structured glucocorticoid taper starting at 10 mg, 20 mg or 30 mg/day [15]. Compared to the patients included in the study by Koster et al., the 15 patients in our series treated with baricitinib had longer disease duration (median [IQR] 32 [12–48] months vs. 9 [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21] months; p = 0.008), less incidence of PMR symptoms at baseline (53% vs. 26%; p = 0.010), significantly higher levels of ESR and CRP at baseline (p ≤ 0.001), and higher rate of prior immunosuppressive treatment failure at the time of starting baricitinib (p < 0.001). As expected given the different study design (prospective clinical trial vs. retrospective real-world study), the prednisone dose at six and twelve months after baricitinib initiation was higher in the patients included in our series. All patients from the study by Koster et al. [15] who completed twelve months of treatment were able to discontinue prednisone, while in our series the median (IQR) daily prednisone dose of the eight patients that received baricitinib for 12 months was 3.7 [0.6–10.6] with only one (14%) patient completely off prednisone by that timepoint (Supplementary Table 1). By week 52, thirteen of the fourteen patients (93%) completing the trial prococol by Koster et al. and five of the eight patients (62%) treated with baricitinib who had reached 52 weeks of in our series were in remission.
Discussion
The results from this observational study and from the literature review indicate that JAKi may be effective in a sizable proportion of GCA patients, including those that previously failed established glucocorticoid-sparing treatments such as methotrexate and tocilizumab. After a median follow up of nearly one year, approximately 60% of the patients receiving JAKi in our series achieved and maintained clinical remission. In addition, the patients in our cohort were able to significantly reduce their daily prednisone doses to a median of 5 mg and 20% of them weaned off glucocorticoids completely.
Glucocorticoids have been the cornerstone of the treatment of GCA for decades. However, relapses are common when glucocorticoid doses are tapered and adverse events from this type of medications are frequent [24]. In addition, glucocorticoids impair quality of life and are negatively regarded in the long-term by most patients with GCA [24]. Over the last several years, other drugs, such as methotrexate and TNF-\(a\) inhibitors have been trialed with controversial or disappointing results [3, 4]. More recently, phase 2 randomized controlled trials with abatacept, mavrilimumab, and secukinumab have shown encouraging preliminary findings that await confirmation [11,12,13]. The only medication thus far with demonstrated efficacy in a phase 3 randomized controlled trial, however, is tocilizumab [6]. Nevertheless, up to one third of patients relapse while on tocilizumab and up to 10% must discontinue treatment due to adverse events [6, 7, 9, 25]. Furthermore, nearly two thirds of patients experience relapses within 12–24 months after tocilizumab discontinuation [8,9,10]. The treatment landscape described above underscores the need for additional GCA therapies.
Macrophages and CD4+ cell with T helper phenotype type 1 (Th1) and 17 (Th17), which are the main immune cell effectors present in GCA lesions, respond to several cytokines through JAK/STAT pathways, and produce cytokines that in turn amplify the inflammatory response in a JAK/STAT-dependent manner creating a vicious cycle [26, 27]. These cytokines include IL-2, IL-12, IL-23, IL-6, GM-CSF, and IFN-\(\gamma\), among others [26, 28,29,30]. Preclinical investigations have shown downregulation of IFN-\(\gamma\), IL-17 and IL-21 leading to decreased CD4+ cell infiltrates, neovascularization and intimal proliferation in response to tofacitinib in a model that utilizes a human artery implant on an immunodeficient humanized mouse. Such model recapitulates GCA-like arterial inflammation upon transfusion with peripheral blood mononuclear cells from GCA patients [14].
Clinical data on the efficacy and safety of JAKi in GCA are scarce and limited to a few cases reports, one small retrospective case series, and one small prospective study (Supplementary Table 2) [15,16,17,18,19,20]. Eriksson et al. [16], evaluated the effectiveness and safety of baricitinib and tofacitinib in 15 relapsing patients with GCA and observed no further relapses, reduction in prednisone use, and improvement in CRP and ESR values during the observation period. Of note, only 20% of these patients had previously received conventional synthetic immunosuppressants and 20% had previously received biologics. Koster et al. [15]. conducted a prospective, open-label, pilot study with baricitinib 4 mg daily for 52 weeks in 15 patients with relapsing GCA. The study employed a pre-specified prednisone taper over 15–22 weeks starting between 10 and 30 mg daily. Fourteen patients completed 52 weeks of treatment and only 1 patient relapsed. The remaining 13 patients achieved and maintained disease remission and were able to discontinue prednisone as per protocol until the end of the study. Noteworthy, only 13% and 7% of the patients enrolled in the trial had previously been treated with conventional synthetic immunosuppressants and biologics, respectively, and the median disease duration of the cohort prior to JAKi therapy was 9 months.
In our series, approximately one third of the patients relapsed while on JAKi and, despite a significant reduction in the daily prednisone dose by the end of follow up, only 20% stopped prednisone completely during the observation period. Possible explanations between our results and the results from the study by Eriksson et al. [16] may include the fact that our cohort of patients could have had more refractory disease reflected by the fact that 63% failed conventional synthetic immunosuppressive agents and 86% failed biological therapy before JAKi initiation. A comparison between our study and the one by Koster et al. [15] is challenging given the markedly different study designs (i.e., retrospective versus prospective), but factors determining what seems to have been an encouraging, yet poorer response to JAKi in our series may also comprise more recalcitrant disease in our cases demonstrated by longer disease duration, and again reflected in the higher exposures to first and second line therapies before JAKi treatment.
Five of the patients treated with JAKi in our series developed adverse events that led to JAKi discontinuation in four of them. Adverse events included two infections (bacterial urinary infection and disseminated varizella-zoster virus infection), one case of liver dysfunction, and one case of glioblastoma multiforme diagnosed after six months of JAKi therapy. No cases of thromboembolism, stroke, myocardial infarction, significant cytopenias, or GCA-related permanent vision loss were observed. Adverse events reported in the study of Eriksson at al [16]. included a case of Aspergillus fumigatus infection and a case Enterococcus faecalis bacteremia. As expected for a prospective clinical trial, over 90% of patients in the study by Koster et al. [15] reported at least one adverse event during the 52 weeks of follow-up. Those adverse events included infection not requiring antibiotics (n = 8), infection requiring antibiotics (n = 5), nausea (n = 6), leg swelling (n = 2), fatigue (n = 2), diarrhea (n = 1), and abdominal pain (n = 1). One patient experienced a severe adverse event consistent of transient thrombocytopenia, which was attributed to concomitant use of antivirals.
The main limitations of our study are its retrospective nature that could have introduced bias due to missing data and the relatively small sample size. In addition, incomplete documentation of data related to individual prednisone tapering courses made the calculation of cumulative prednisone dose, a key outcome measure in GCA, inaccurate and therefore not analyzable. Despite these limitations, information about key efficacy and safety events (e.g., remission, relapse, serious adverse events, and drug discontinuation) were unequivocally present in the data source, which makes our estimations reliable. Moreover, to our knowledge, this is the largest study to date evaluating outcomes of GCA patients treated with JAKi. As such, our results expand prior findings [15, 16], which have been mostly limited to patients naïve to treatment with conventional synthetic immunosuppressants and biologics, suggesting that JAKi can be useful in patients failing those therapies as well.
Conclusion
In summary, in this retrospective study, JAKi treatment was associated with GCA disease control including reduction in the prednisone use in a sizable proportion of patients, most of whom had failed established glucocorticoid-sparing options including tocilizumab and methotrexate. Until the results of a large phase 3 randomized-controlled trial with upadacitinib (Clinical Trials.gov identifier NCT03725202) become available, our findings may inform clinical decision making for GCA patients in routine practice.
Data availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are included in the paper.
Abbreviations
- CRP:
-
C-reactive protein
- CTA:
-
Computed tomography angiography
- ESR:
-
Erythrocyte sedimentation rate
- EULAR:
-
European League Against Rheumatism
- FDG:
-
Fluorodeoxyglucose
- GCA:
-
Giant cell arteritis
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- IL-6R:
-
IL-6 receptor
- IFN:
-
Interferon
- IQR:
-
Interquartile range
- JAKi:
-
JAK inhibitors
- JAK/STAT:
-
Janus kinase/signal transducers and activators of transcription
- MRI/MRA:
-
Magnetic resonance imaging/angiography
- PET:
-
Positron emission tomography
- PMR:
-
Polymyalgia rheumatica
- SD:
-
Standard deviation
- Th1:
-
T helper type 1
- Th17:
-
T helper type 17
- TNF:
-
tumor necrosis factor
References
Salvarani C, Cantini F, Hunder GG. Polymyalgia Rheumatica and giant-cell arteritis. Lancet. 2008;372:234–45.
Loricera J, Blanco R, Hernández JL, et al. Use of positron emission tomography (PET) for the diagnosis of large-vessel vasculitis. Rev Esp Med Nucl Imagen Mol. 2015;34:372–7.
Narváez J, Estrada P, Llop D, et al. Efficacy and safety of leflunomide in the management of large vessel vasculitis: a systematic review and metaanalysis of cohort studies. Semin Arthritis Rheum. 2023;59:152166.
Loricera J, Blanco R, Hernández JL, et al. Biologic therapy in ANCA-negative vasculitis. Int Immunopharmacol. 2015;27:213–9.
Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomized, double-blind, placebo-controlled trial. Lancet. 2016;387:1921–7.
Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377:317–28.
Unizony SH, Bao M, Han J, Luder Y, Pavlov A, Stone JH. Treatment failure in giant cell arteritis. Ann Rheum Dis. 2021;80:1467–74.
Stone JH, Aringer M, Blockmans D, et al. Long-term effect of tocilizumab in patients with giant cell arteritis: open-label extension phase of the Giant Cell Arteritis Actemra (GiACTA) trial. Lancet Rheumatol. 2021;3:e328–36.
Matza MA, Dagincourt N, Mohan SV, et al. Outcomes during and after long-term tocilizumab treatment in patients with giant cell arteritis. RMD Open. 2023;9:e002923.
Samec MJ, Rakholiya J, Langenfeld H, et al. Relapse risk and safety of long-term tocilizumab use among patients with giant cell arteritis: a single-enterprise cohort study. J Rheumatol. 2023;5:341–50.
Venhoff N, Schmidt WA, Bergner R, et al. Safety and efficacy of secukinumab in patients with giant cell arteritis (TitAIN): a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Rheumatol. 2023;5:E341–50.
Cid MC, Unizony SH, Blockmans D, et al. Efficacy and safety of mavrilimumab in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2022;81:653–61.
Langford CA, Cuthbertson D, Ytterberg SR, et al. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol. 2017;69:837–45.
Zhang H, Watanabe R, Berry GJ, Tian L, Goronzy JJ, Weyand CM. Inhibition of JAK-STAT signaling suppresses pathogenic immune responses in medium and large vessel vasculitis. Circulation. 2018;137:1934–48.
Koster MJ, Crowson CS, Giblon RE, et al. Baricitinib for relapsing giant cell arteritis: a prospective open-label 52-week pilot study. Ann Rheum Dis. 2022;81:861–67.
Eriksson P, Skoglund O, Hemgren C, Sjöwall C. Clinical experience and safety of Janus kinase inhibitors in giant cell arteritis: a retrospective case series from Sweden. Front Immunol. 2023;14:1187584.
Prigent K, Aouba A, Aide N, de Boysson H. JAK inhibitor effectiveness in giant-cell arteritis with large-vessel involvement assessed by 18F-FDG PET-CT. Clin Nucl Med. 2022;47:234–35.
Herlihy N, Curto-García N, O´Sullivan J, et al. Successful treatment of chronic neutrophilic leukaemia and associated giant cell arteritis with the combination of ruxolitinib and azacytidine [abstract]. Br J Haematol. 2019;185:71–2.
Camellino D, Dejaco C, Giusti A, et al. Baricitinib in Polymyalgia Rheumatica and giant cell arteritis: report of six cases [abstract]. Ann Rheum Dis. 2021;80:1216.
Sanada A, Abe N, Bohgaki M, Kasahara H. Therapeutic effectiveness of upadacitinib combined with glucocorticoid on remission induction and maintenance in giant cell arteritis. Rheumatology (Oxford). 2022;61:e274–6.
Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–8.
Dejaco C, Kerschbaumer A, Aletaha D et al. Treat-to-target recommendations in giant cell arteritis and polymyalgia rheumatica. Ann Rheum Dis 2023; ard-2022-223429.
Maz M, Chung SA, Abril A, et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis Rheumatol. 2021;73:1349–65.
Wilson JC, Sarsour K, Collinson N, et al. Serious adverse effects associated with glucocorticoid therapy in patients with giant cell arteritis (GCA): a nested case-control analysis. Semin Arthritis Rheum. 2017;46:819–27.
Miyabe C, Miyabe Y, Strle K, et al. An expanded population of pathogenic regulatory T cells in giant cell arteritis is abrogated by IL-6 blockade therapy. Ann Rheum Dis. 2017;76:898–905.
Bursi R, Cafaro G, Perricone C, et al. Contribution of Janus-Kinase/Signal Transduction Activator of Transcription Pathway in the pathogenesis of Vasculitis: a possible treatment target in the Upcoming Future. Front Pharmacol. 2021;12:635663.
Castelo-Soccio L, Kim H, Gadina M, Schwartzberg PL, Laurence A, O´Shea JJ. Protein kinases: drug targets for immunological disorders. Nat Rev Immunol. 2023;15:1–20.
Watanabe R, Berry GJ, Liang DH, Goronzy JJ, Weyand CM. Cellular signaling pathways in medium and large vessel vasculitis. Front Immunol. 2020;11:587089.
Schwartz DM, Bonelli M, Gadina M, O´Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016;12:25–36.
Terrades-Garcia N, Cid MC. Pathogenesis of giant-cell arteritis: how targeted therapies are influencing our understanding of the mechanisms involved. Rheumatology (Oxford). 2018;57:51–62.
Acknowledgements
We thank all the members of the different hospitals and patients included in this study.
Funding
No specific funding was received from any bodies in the public, commercial or non-for-profit sectors to carry out the work described in this article.
Author information
Authors and Affiliations
Contributions
JL, TT, SC, SU and RB conceptualized, and designed the study. SU and RB were the research coordinators for the study. RB was the project manager for the study. IF-A supervised the study. JL, TT, DP-P, SR-Y, EM, AR-F, EL, OM, EB, JN, EG-A, IG-F, AU-A, AR-C, FL-G, SC and SU collected data. JL, IF-A, SC, SU and RB curated the data. JL, IF-A, SC, SU and RB analysed the data. JL, IF-A, SC, SU and RB interpreted the data. JL wrote the original manuscript. JL, TT, DP-P, SR-Y, EM, AR-F, EL, OM, EB, JN, EG-A, IG-F, AU-A, AR-C, FL-G, SC, SU and RB reviewed and edited the original manuscript. JL and RB had direct access to the data and verified the underlying raw data reported in the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Cantabria Clinical Research Ethics Committee (approval number 2021.414), and was conducted in accordance with the Declaration of Helsinki and the International Conference for Harmonization. All data extracted from the medical records were stored de-identified prior to the analysis. As per the Clinical Research Ethics Committee, this retrospective research did not require informed consent.
Consent for publication
Not applicable.
Competing interests
Disclosures that might be interpreted as constituting possible conflict(s) of interest for the study: Javier Loricera had consultation fees/participation in company-sponsored speaker´s bureau from Roche, AbbVie, Galápagos, Novartis, UCB Pharma, MSD, Celgene, Astra Zeneca, and Grünenthal and received support for attending meetings and/or travel from Janssen, AbbVie, Roche, Novartis, MSD, UCB Pharma, Celgene, Lilly, Pfizer, and Galápagos. Diana Prieto-Peña has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from UCB Pharma, Novartis, Amgen, Lilly, Sanofi, and Janssen, and received support for attending meetings and/or travel from Lilly, Roche, Sanofi, Pfizer and Abbvie. Susana Romero-Yuste has received grants/recents supports from Abbvie, Lilly, Pfizer, and Galápagos. Eugenio de Miguel Research funding/consulting and conferences fees from: Abbvie, Novartis, Pfizer, Roche, Janssen, Lilly, MSD, BMS, UCB, Grünenthal, and Sanofi, and received support for attending meetings and/or travel from Abbvie, Pfizer, Sanofi, and Grünenthal. Iván Ferraz-Amaro would like to acknowledge that he has received grants/research supports from Abbvie, MSD, Janssen, and Roche, as well as consultation fees from company sponsored speakers bureaus associated with Abbvie, Pfizer, Roche, Sanofi, Celgene, and MSD, and received support for attending meetings and/or travel from Abbvie, MSD, Janssen, Pfizer, Roche, Sanofi, and Celgene. Eva Galíndez-Agirregoikoa had consultation fees/participation in company-sponsored speaker´s bureau from Lilly, Janssen, Abbvie, and Amgen, and received support for attenting meetings and/or travel from Lilly, Abbvie, and Pfizer. Ismael González-Fernández had consultation fees/participation in company-sponsored speaker´s bureau from Novartis, Janssen, Grunenthal, MSD, and Theramex, and received support for attending meetings and/or travel from Janssen, and MSD. Ana Urruticoechea-Arana had received research grants, fees for consultancies or presentations and participated in medical meetings or courses from Abbvie, Galápagos, UCB, Pfizer, MSD, Novartis, GSK, Hanssen, and Amgen, and participated on a data safety monitoring board or advisory board GSK. Ángel Ramos-Calvo had consultation fees/participation in company-sponsored speaker´s bureau from Amgen, UCB, and Galápagos, and received support for attending meetings and/or travel from Pfizer. Fernando López-Gutiérrez received support for attending meetings and/or travel from Janssen, Abbvie, Roche, Novartis, MSD, UCB Pharma, Celgene, Lilly, Pfizer, and Galápagos. Santos Castañeda has received research supports from MSD, and Pfizer and had consultation fees/participation in company-sponsored speaker’s bureau from BMS, Eli-Lilly, MSD, Roche, and UCB, and received support for attending meetings and/or travel from BMS, Lilly, MSD, Roche, and UCB. Sebastian Unizony received research support from Genentech, participated in an advisory board for Abbvie, and provided consulting for Sanofi, Kiniksa, and Janssen.Ricardo Blanco received grants/research support from AbbVie, MSD, and Roche, and had consultation fees/participation in a company-sponsored speaker’s bureau from AbbVie, Pfizer, Roche, GSK, Lilly, UCB, Bristol-Myers, Novartis, Janssen, UCB and MSD, and received support for attending meetings and/or travel from AbbVie, Pfizer, Roche, GSK, Lilly, UCB, Bristol-Myers, Novartis, Janssen, UCB, and MSD. The following authors did not declare financial disclosure: Toluwalase Tofade, Anne Riveros-Frutos, Eztizen Labrador, Olga Maiz, Elena Becerra, Javier Narváez. The following authors did not declare financial disclosure: Toluwalase Tofade, Anne Riveros-Frutos, Eztizen Labrador, Olga Maiz, Elena Becerra, Javier Narváez.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Loricera, J., Tofade, T., Prieto-Peña, D. et al. Effectiveness of janus kinase inhibitors in relapsing giant cell arteritis in real-world clinical practice and review of the literature. Arthritis Res Ther 26, 116 (2024). https://doi.org/10.1186/s13075-024-03314-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-024-03314-9